LEAP™ Advanced Therapy Platform
Accelerate Your Path to Clinic with BioCentriq’s LEAP™ Platform
The LEAP™ platform offered by BioCentriq provides early-stage biotech companies with novel cell therapy candidates an avenue to leverage proven expertise and assets to reach patients in record time.
Sponsors who leverage LEAP™ minimize up-front investment in process development, reduce risk, accelerate timelines, and lower the cost of IND-enabling studies and clinical production.
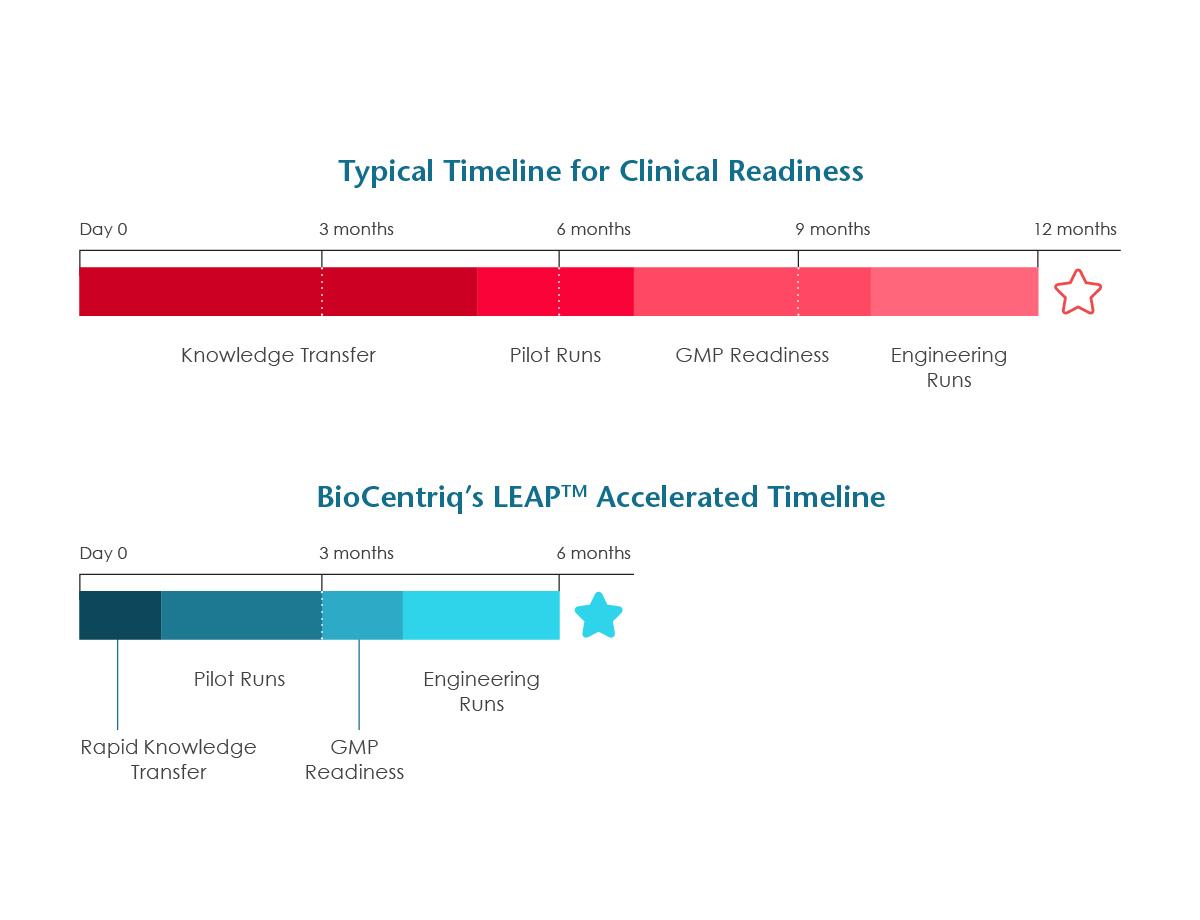
Get to Clinic in Six Months
Leverage BioCentriq Assets
Moving from project kick-off to patient dosing in six months is achieved using BioCentriq’s established methods and processes for scale up, including:
- Pre-Existing Master Batch Records
- Established SOPs
- Qualified Analytical Methods
- Trained Personnel
- Completed Aseptic Validation
- Proven and Reliable Supply Chain
- Phase Appropriate Quality System
- Fit-for-Purpose Facilities and Equipment
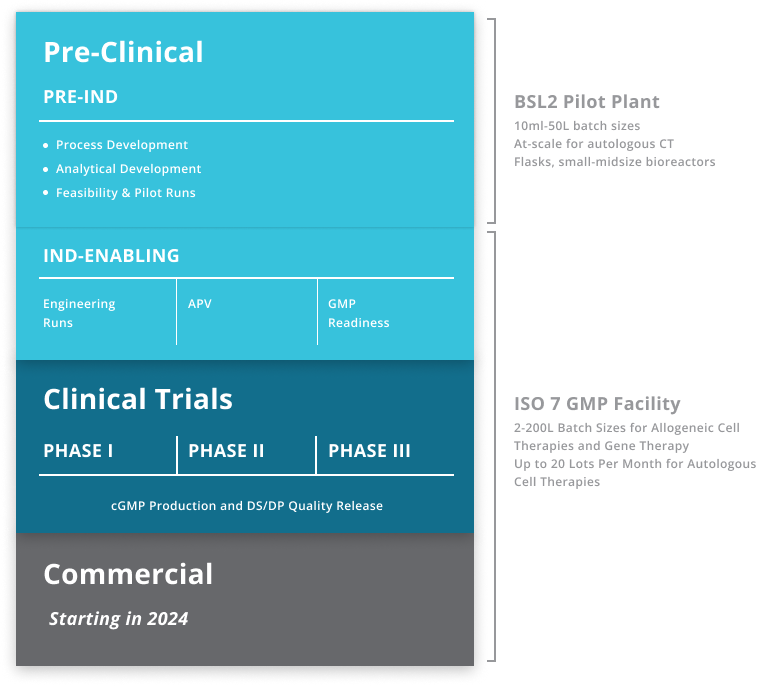
LEAP™ Advanced Therapy Platform for Multiple Modalities
BioCentriq can offer the LEAP™ platform in the following modalities:
- LEAP-NK™
- LEAP-CAR™
- LEAP-TCELL™
- LEAP-TREG™
- LEAP-MSC™
- LEAP-IPSC™
- LEAP-DC™
- LEAP-EXOSOME™
- LEAP-MACRO™
Benefits of Leveraging BioCentriq’s LEAP™ Platform
- Reduce process development and scale-up timelines by up to 75%
- De-risk clinical trials with proven manufacturing processes and analytical methods
- Move quickly by capitalizing on existing aseptic validation and facility and equipment qualifications
- Utilize pre-existing documentation including protocols, methods, reports and batch records
- Access a qualified team with verified experience and recognized expertise
Why Choose BioCentriq?
- Experienced and expert team
- Pre-qualified equipment, methods and facilities
- Verified ability to scale-up
- Collaborative and transparent approach
- Willingness to integrate sponsor expertise either on-site or virtually
- Phase appropriate quality systems
- Track record of producing product for use in human clinical trials
"We're extremely enthusiastic about the promising results presented in our abstract, "Large-scale biomanufacturing platform to produce high-quality, therapeutically relevant NK cells using the LEAP-NK™ Platform." The results suggest BioCentriq’s LEAP-NK™ platform utilizing propriety feeder cell lines can enhance expansion of NK cells >12,000 fold."

Mary Loveras
"At BioCentriq, we understand the complexities of navigating a funding-constrained environment, which is why we offer Rapid Tech Transfer, a risk-based approach to help therapy developers prioritize speed, reach milestones, and ultimately, get their therapeutics to patients in need."

Melissa Mastro
Senior MS&T Engineer