Clinical Manufacturing
High Quality, Efficient & Reliable Manufacturing
Our GMP manufacturing team is expert at producing high quality cell therapy products for use in clinical trials. The team consists of qualified aseptic operators who have years of experience in biologic manufacturing and have been carefully trained and onboarded by the BioCentriq team.
Manufacturing is supported by an experienced and knowledgeable supply chain management team who have established vendor relationships and will efficiently and expertly source the materials required for your program. We also have investigators who will facilitate the assessment of any deviations that occur and a training team that ensures operators assigned to your program are trained in project specific unit operations and qualified to support your process.
Why Choose BioCentriq?
Our hybrid model allows you to observe or work alongside our operators.
BioCentriq's in-house training team develops skilled and qualified operators.
Our global vendor relationships are leveraged to address supply chain challenges.
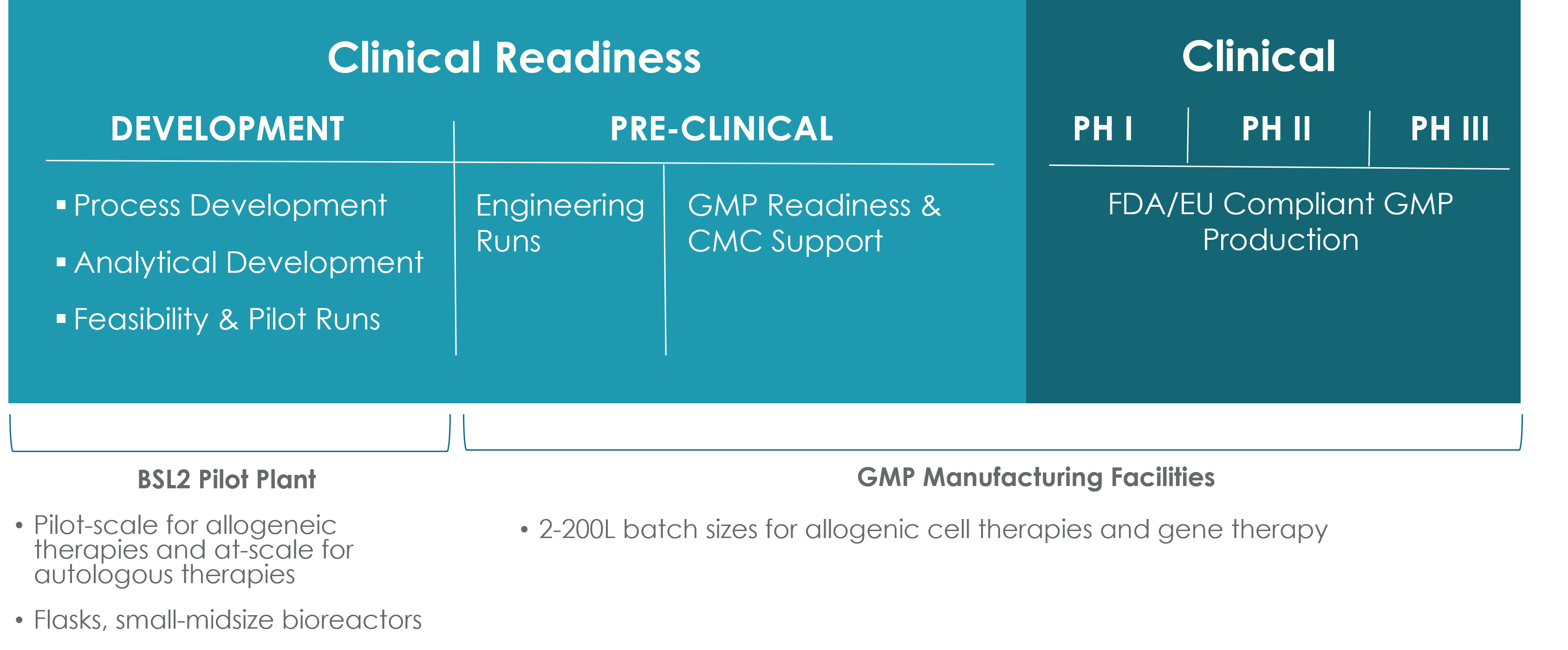
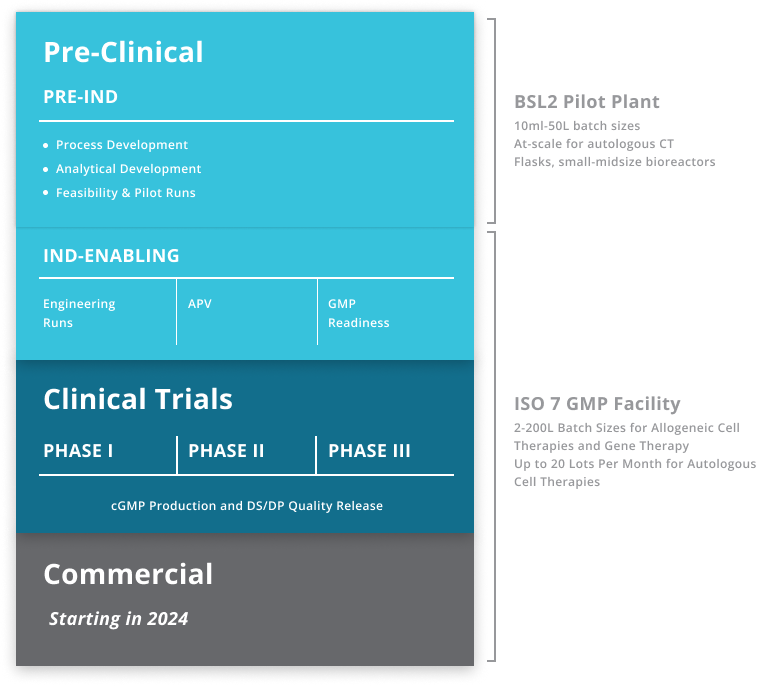
What Phases of Clinical Manufacturing Does BioCentriq Support?
Key Features of Our Manufacturing Capability
The BioCentriq Team
The manufacturing team at BioCentriq includes highly experienced operators who are qualified in aseptic technique, GMP procedures and GDP. In addition, we are fortunate to have seasoned leaders and supervisors who bring significant value to all our clients.
BioCentriq's New Jersey location gives it superior access to a pool of trained personnel coming from nearby big pharmaceutical companies who are downsizing or transitioning their manufacturing.
Learn more about our people.
Newly Constructed, Modern Facilities
BioCentriq has two state-of-the-art, newly constructed facilities for cGMP manufacturing.
We have two clean room manufacturing suites and supporting infrastructure in Newark, NJ and two more suites in South Brunswick, NJ which also houses our pilot plant, analytical method development and QC laboratory.